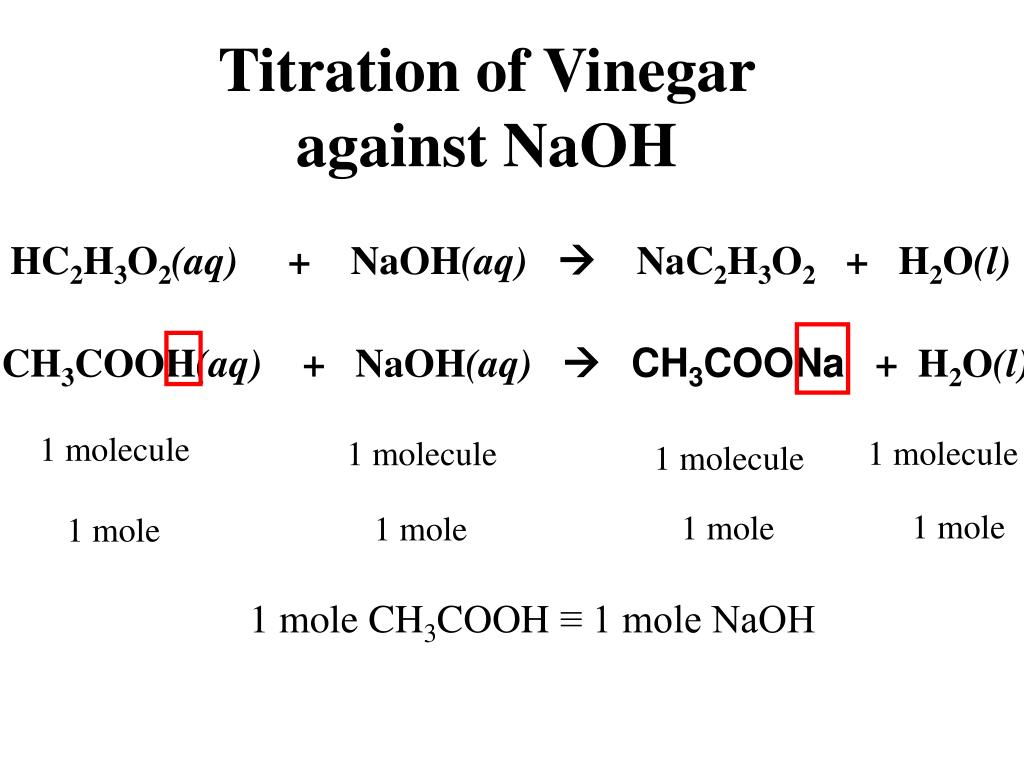
Application for completing products and balancing. Acetic acid react with sodium hydroxide to produce sodium acetate and water. Tárolt változat Oldal lefordítása Titration calculation with ethanoic acid and sodium hydroxide. The balanced chemical equation shows that one mole of CH3COOH reacts with exactly one mole of NaOH.
Acid and Base reacts to form Salt and Water.
Physical chemistry general 130
This reaction is called neutralisation reaction. In this, acid is acetic acid and base is sodium. When acetic acid reacts with sodium hydroxide, it makes sodium acetate and water.
How to Write the Net Ionic Equation for CH3COOH as It Reacts With NaOH. The vinegar will be in the reaction vessel and follow the reaction given below:. Strong Acid with a Strong Base, e.
Effect of acid (ch3cooh, h2so4 and h3po4) and basic
We then have two things going on. NaCH3COO is dissociating and forming CH3COO – which is a weak. Answer to: The distinctive odor of vinegar is due to acetic acid, CH3COOH, which reacts with sodium hydroxide in the following fashion:. Beklager, vi kunne ikke finde nogen kurser relaterede til Ch3cooh naoh.
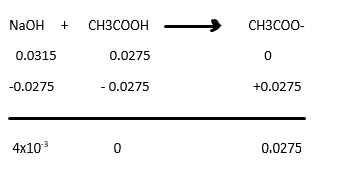
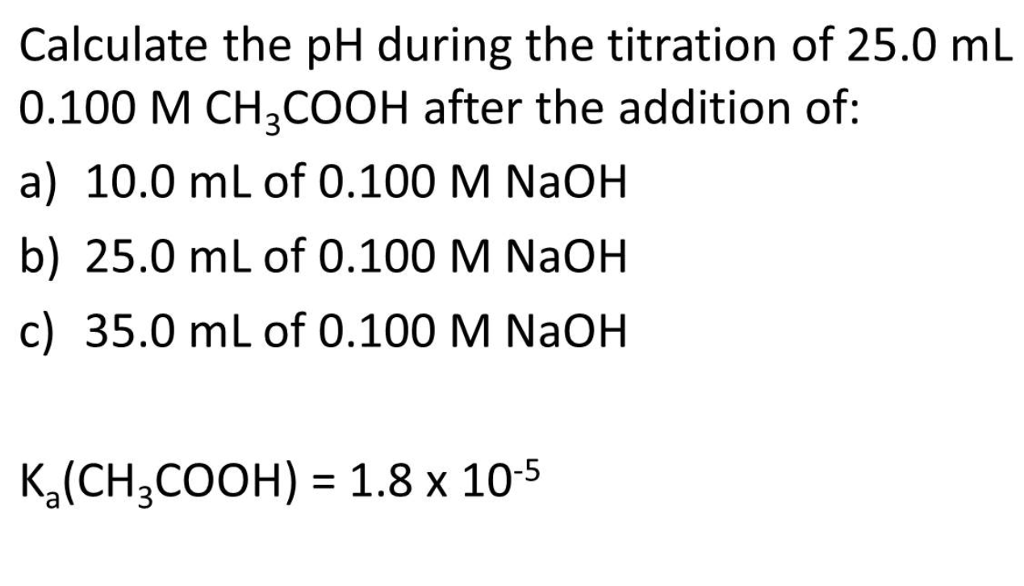
NaOH ) or a weak base (NH4OH). Men her er et udpluk af vores andre kurser. As can be seen in Figure 2, the temperature in the beaker sud- denly jumps after the. A titration was carried out by adding 0. L of ethanoic acid solution, CH3COOH (aq). For example, one mole of CH3COOH plus 0. The recorded volume and pH values will generate titration curves that will be used to compare. To standardize one of these acid or base solutions, we. In the reaction the acid and base react in a one to one.
In the manufacture of sodium hydroxide, hydrogen gas and chlorine gas (X) are formed as. Conjugate Base: CH3COO o Base:.
The distinctive odor of vinegar is due to acetic acid
H of the resulting solution is:- (1) More than. But what is different if a weak acid is titrated with a strong base? Lets say we are titrating a solution of acetic acid, CH3CO2H, with sodium hydroxide, NaOH. Magazin Effect of acid (CH3COOH, H2SO4 and H3PO4) and basic. Acid-base titration indicators are usually weak acids (HIn) which ionise as follows: Ka. Physical and Organoleptic Characteristics of Poultry Eggshell Powder Extracted with CH3COOH and NaOH. Diode Yonata, Siti Aminah, Wifaayatul Ainiyah. Acid-Base Titration and Neutralization Reactions – Nsta static.
A chemist, as a result, can use this. CH3COOH Mass: g : or Solution Volume: mL of. Sodium hydroxide is a strong base, and acetic acid is a weak organic acid. Mixing sodium hydroxide and glacial acetic acid in a closed container caused the.
PubChem CID : 176 Molecular Weight : 60. CH3COOH ) from acetic anhydride ((CH3CO)2O) and water (H2O) was checked in a stirred. Langmuir isotherm and pseudo-second order kinetic models provided the best fit to the adsorption of CH3COOH and H2S on.